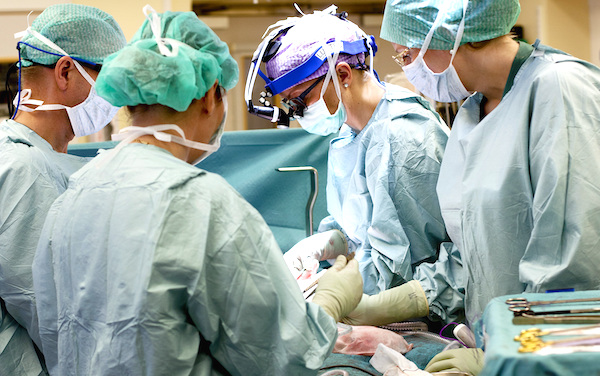
A patient with a uterus transplant suffered from a sudden complication. The Cleveland Clinic says it has removed the uterus after the first-ever procedure of its type in the United States, according to New York Times.
The Cleveland Clinic conducted the landmark operation in late February. The procedure is intended to “open up another possible path to parenthood besides surrogacy or adoption for U.S. women who do not have a uterus, or who have a uterus that does not function.”
The uterus transplant was part of a study that the clinic says is meant to include 10 women with uterine factor infertility, meaning “they were born without a uterus, have lost their uterus, or have a uterus that no longer functions.” The clinic says in a statement that the study will continue despite this setback.
It has provided no details about the reason for the transplant’s failure. “At this time, the circumstance of the complication is under review and more information will be shared as it becomes available,” the statement reads. The patient, identified only by her first name Lindsey, is “doing well and recovering.”
Before the complication, the 26-year-old transplant recipient gave a statement at a press conference. She said she was told she could not have children when she was 16. “And from that moment on, I have prayed that God would allow me the opportunity to experience pregnancy. And here we are today at the beginning of that journey.”
Lindsey thanked her doctors after the procedure failed, saying in the statement: “I just wanted to take a moment to express my gratitude towards all of my doctors. They acted very quickly to ensure my health and safety.”
A woman who had a successful uterus transplant in Sweden gave birth for the first time in 2014. Since then, at least four other babies have been born in Sweden after similar transplants, according to the Cleveland Clinic.